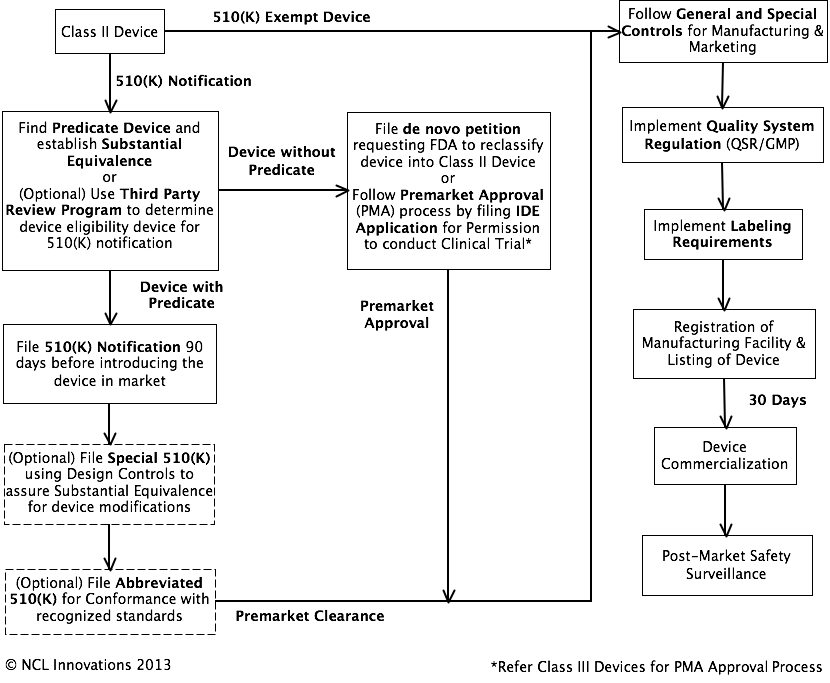
USFDA Regulation for Class II
Devices
· Class II devices are subject to general and special controls, which may include special labeling requirements, mandatory performance standards and post-market safety surveillance.
· Most Class II devices are approved under a 510(K) premarket notification submission except for devices listed in Reserved Medical Devices.
· New devices without an equivalent in market are approved through de novo process.
References:
2) USFDA General Controls for
Class I Devices.
3)
Quality
System Regulation (QSR)/ Good Manufacturing Practices (GMP)
5)
Registration
of Manufacturing Facility & Listing of Devices
10) Special 510(K)
12) De Novo Classification Process
13) Investigational Device Exemption (IDE)
14) Premarket Approval Application (PMA)
15) Expedited Review of Devices Subject to PMA
16) Post-Market Safety Surveillance – Reporting Adverse Events
17) Modifications to Devices Subject to 510(K)
18) Modifications to Devices Subject to PMA